Background
Recent advances have led to higher response rates and improved survival in patients with newly diagnosed AML. However, early death, lack of response, and long term leukemia-free survival remain important challenges. Early mortality in AML has been reported to be as high as 20-30%. Similar to relapse risk, disease related factors (along with patient and treatment factors) may also contribute significantly to early mortality. Here we investigated predictors of early (4- and 8-week) mortality, response, relapse-free survival, and overall survival in a contemporary cohort from a single large academic medical center.
Methods
We analyzed all newly diagnosed patients with AML presenting to and treated at our institution from January 2012 to January 2020. For 4- and 8-week mortality and overall response rate (CR/CRi), logistic regression models were generated to identify factors associated with these outcomes. Kaplan-Meier methods were used to determine the median of the time to event outcomes. Cox proportional hazards modelswere used to identify any association with each of the variables and survival outcomes. Multivariate models were performed for all the outcomes, the models included variables with p<0.25 in the univariate analysis. Backward elimination methods were used to determine a final multivariate models for each outcome.
Results
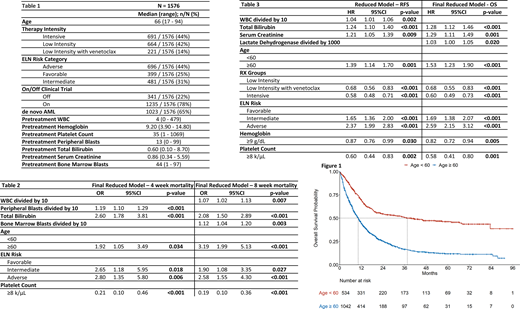
Our cohort included 1576 consecutive patients (pts) with newly diagnosed AML with a median follow-up of 11 months (range 0 - 96). The median age is 66 (range: 17 - 94), with 66% of pts being ≥ 60 years (yrs) old. By 2017 ELN risk classification, 44% were classified as adverse, 31% intermediate, and 25% were favorable risk. To simplify induction regimens, we classified patients as receiving intensive (high dose Ara-C based), low intensity, and low intensity with venetoclax. 44% received intensive, 42% received low intensity, and 14% received low intensity with venetoclax therapy.
The 4- and 8-week mortality rates were 5% and 11%, respectively. Among pts < 60 yrs the 4- and 8 week mortality rates were 3% and 5%, respectively; while those rates in pts ≥ 60 yrs were 6% and 14%, respectively. In the final reduced models the following factors are associated with both increased 4- and 8-week mortality: elevated baseline total bilirubin, age ≥ 60, and ELN intermediate and adverse risk classifications, when compared to ELN favorable. Elevated baseline WBC and bone marrow blasts were associated with 8-week but not 4-week mortality. Baseline platelet count ≥ 8k/µL was associated with reduced 4 and 8-week mortality. Among 97 pts who died between 4 and 8 weeks 87 patients (90%) had active disease or no response to their initial therapy, suggesting disease refractoriness as an important contributor to early death.
The factors associated with decreased CR/CRi were increased baseline peripheral blasts, total bilirubin, serum creatinine, age ≥ 60, and ELN risk classifications when compared to favorable risk disease. Treatment with low intensity plus venetoclax and intensive therapy were associated with increased CR/CRi rates as was baseline platelet count ≥ 8k/µL.
Median relapse-free survival for the cohort is 8 months (95% CI: 7- 9). Median overall survival for the cohort is 13 months (95% CI: 12- 14), with median OS for those ≥ 60 yrs: 10months (95%CI: 9- 11) and those < 60 yrs: 37months (95%CI: 24- 67). Factors associated with increased hazard of both relapse and death were increased baseline total bilirubin, creatinine, age ≥ 60, and ELN risk classifications when compared favorable disease. Factors associated with decreased hazard of both relapse and death were treatment with low intensity plus venetoclax and intensive therapy when compared to low intensity, and baseline hemoglobin ≥ 9 g/dl and platelet count ≥ 8k/µL.
Conclusion
In this study, using a contemporary cohort of consecutive, unselected AML patients, we identified parameters associated with early mortality, response, relapse-free, and overall survival. Compared to published historical data, patients at a high-volume academic center experienced lower early mortality and favorable long term outcomes. We identify both patient (i.e. baseline organ dysfunction) and disease (i.e. ELN risk) intrinsic characteristics that have a significant impact clinical outcomes in patients with AML. Further analysis including the impact mutational groups outside of ELN and treatment on or off investigational trials will be presented.
Ravandi:Xencor: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Macrogenics: Research Funding; BMS: Consultancy, Honoraria, Research Funding; Orsenix: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; Astellas: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding. Sasaki:Novartis: Consultancy, Research Funding; Pfizer Japan: Consultancy; Otsuka: Honoraria; Daiichi Sankyo: Consultancy. Borthakur:FTC Therapeutics: Consultancy; BioLine Rx: Consultancy; BioTherix: Consultancy; Nkarta Therapeutics: Consultancy; Treadwell Therapeutics: Consultancy; Curio Science LLC: Consultancy; Oncoceutics: Research Funding; Xbiotech USA: Research Funding; Polaris: Research Funding; AstraZeneca: Research Funding; BMS: Research Funding; BioLine Rx: Research Funding; Cyclacel: Research Funding; GSK: Research Funding; Jannsen: Research Funding; Abbvie: Research Funding; Novartis: Research Funding; Incyte: Research Funding; PTC Therapeutics: Research Funding; PTC Therapeutics: Consultancy; Argenx: Consultancy. Garcia-Manero:Bristol-Myers Squibb: Consultancy, Research Funding; Astex Pharmaceuticals: Consultancy, Honoraria, Research Funding; Onconova: Research Funding; Acceleron Pharmaceuticals: Consultancy, Honoraria; H3 Biomedicine: Research Funding; AbbVie: Honoraria, Research Funding; Helsinn Therapeutics: Consultancy, Honoraria, Research Funding; Novartis: Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amphivena Therapeutics: Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy; Merck: Research Funding. Daver:Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Research Funding; Servier: Research Funding; Genentech: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novimmune: Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Trovagene: Research Funding; Fate Therapeutics: Research Funding; ImmunoGen: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Trillium: Consultancy, Membership on an entity's Board of Directors or advisory committees; Syndax: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. DiNardo:Novartis: Consultancy; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Agios: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Jazz: Honoraria; ImmuneOnc: Honoraria; AbbVie: Consultancy, Honoraria, Research Funding; Syros: Honoraria; MedImmune: Honoraria; Takeda: Honoraria; Calithera: Research Funding; Notable Labs: Membership on an entity's Board of Directors or advisory committees. Jabbour:Pfizer: Other: Advisory role, Research Funding; Genentech: Other: Advisory role, Research Funding; BMS: Other: Advisory role, Research Funding; Adaptive Biotechnologies: Other: Advisory role, Research Funding; AbbVie: Other: Advisory role, Research Funding; Amgen: Other: Advisory role, Research Funding; Takeda: Other: Advisory role, Research Funding. Pemmaraju:AbbVie: Honoraria, Research Funding; Incyte Corporation: Honoraria; Novartis: Honoraria, Research Funding; MustangBio: Honoraria; Daiichi Sankyo: Research Funding; SagerStrong Foundation: Other: Grant Support; DAVA Oncology: Honoraria; Cellectis: Research Funding; Plexxikon: Research Funding; Samus Therapeutics: Research Funding; Stemline Therapeutics: Honoraria, Research Funding; Pacylex Pharmaceuticals: Consultancy; LFB Biotechnologies: Honoraria; Affymetrix: Other: Grant Support, Research Funding; Blueprint Medicines: Honoraria; Roche Diagnostics: Honoraria; Celgene: Honoraria. Andreeff:Centre for Drug Research & Development; Cancer UK; NCI-CTEP; German Research Council; Leukemia Lymphoma Foundation (LLS); NCI-RDCRN (Rare Disease Clin Network); CLL Founcdation; BioLineRx; SentiBio; Aptose Biosciences, Inc: Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo; Breast Cancer Research Foundation; CPRIT; NIH/NCI; Amgen; AstraZeneca: Research Funding; Amgen: Research Funding; Daiichi-Sankyo; Jazz Pharmaceuticals; Celgene; Amgen; AstraZeneca; 6 Dimensions Capital: Consultancy. Verstovsek:Incyte Corporation: Consultancy, Research Funding; Roche: Research Funding; AstraZeneca: Research Funding; PharmaEssentia: Research Funding; Sierra Oncology: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Blueprint Medicines Corp: Research Funding; Gilead: Research Funding; Promedior: Research Funding; Genentech: Research Funding; CTI Biopharma Corp: Research Funding; ItalPharma: Research Funding; Protagonist Therapeutics: Research Funding; Celgene: Consultancy, Research Funding; NS Pharma: Research Funding. Jain:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Cellectis: Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Research Funding; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Research Funding; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding; BeiGene: Honoraria, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Research Funding; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Precision Bioscienes: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Fate Therapeutics: Research Funding; Aprea Therapeutics: Research Funding; TG Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees. Konopleva:AbbVie: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Stemline Therapeutics: Consultancy, Research Funding; Sanofi: Research Funding; Rafael Pharmaceutical: Research Funding; Reata Pharmaceutical Inc.;: Patents & Royalties: patents and royalties with patent US 7,795,305 B2 on CDDO-compounds and combination therapies, licensed to Reata Pharmaceutical; Eli Lilly: Research Funding; Agios: Research Funding; Ablynx: Research Funding; Forty-Seven: Consultancy, Research Funding; Amgen: Consultancy; Calithera: Research Funding; Kisoji: Consultancy; Cellectis: Research Funding; AstraZeneca: Research Funding; F. Hoffmann La-Roche: Consultancy, Research Funding; Ascentage: Research Funding. Kantarjian:Abbvie: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Ascentage: Research Funding; BMS: Research Funding; Daiichi-Sankyo: Honoraria, Research Funding; Immunogen: Research Funding; Jazz: Research Funding; Novartis: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Sanofi: Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive biotechnologies: Honoraria; Aptitute Health: Honoraria; BioAscend: Honoraria; Delta Fly: Honoraria; Janssen: Honoraria; Oxford Biomedical: Honoraria. Kadia:Cyclacel: Research Funding; Novartis: Honoraria; Incyte: Research Funding; Genentech: Honoraria, Research Funding; Cellenkos: Research Funding; Ascentage: Research Funding; Pulmotec: Research Funding; Astra Zeneca: Research Funding; Celgene: Research Funding; Abbvie: Honoraria, Research Funding; JAZZ: Honoraria, Research Funding; BMS: Honoraria, Research Funding; Astellas: Research Funding; Amgen: Research Funding; Pfizer: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal